Pharmaceutical stability testing is the process by which drug manufacturers collect data on their product over predetermined lengths of time in specific environmental conditions to determine if there is any change in the quality of the Active Pharmaceutical Ingredient (API) or Final Product (FP). The data collected from stability studies.
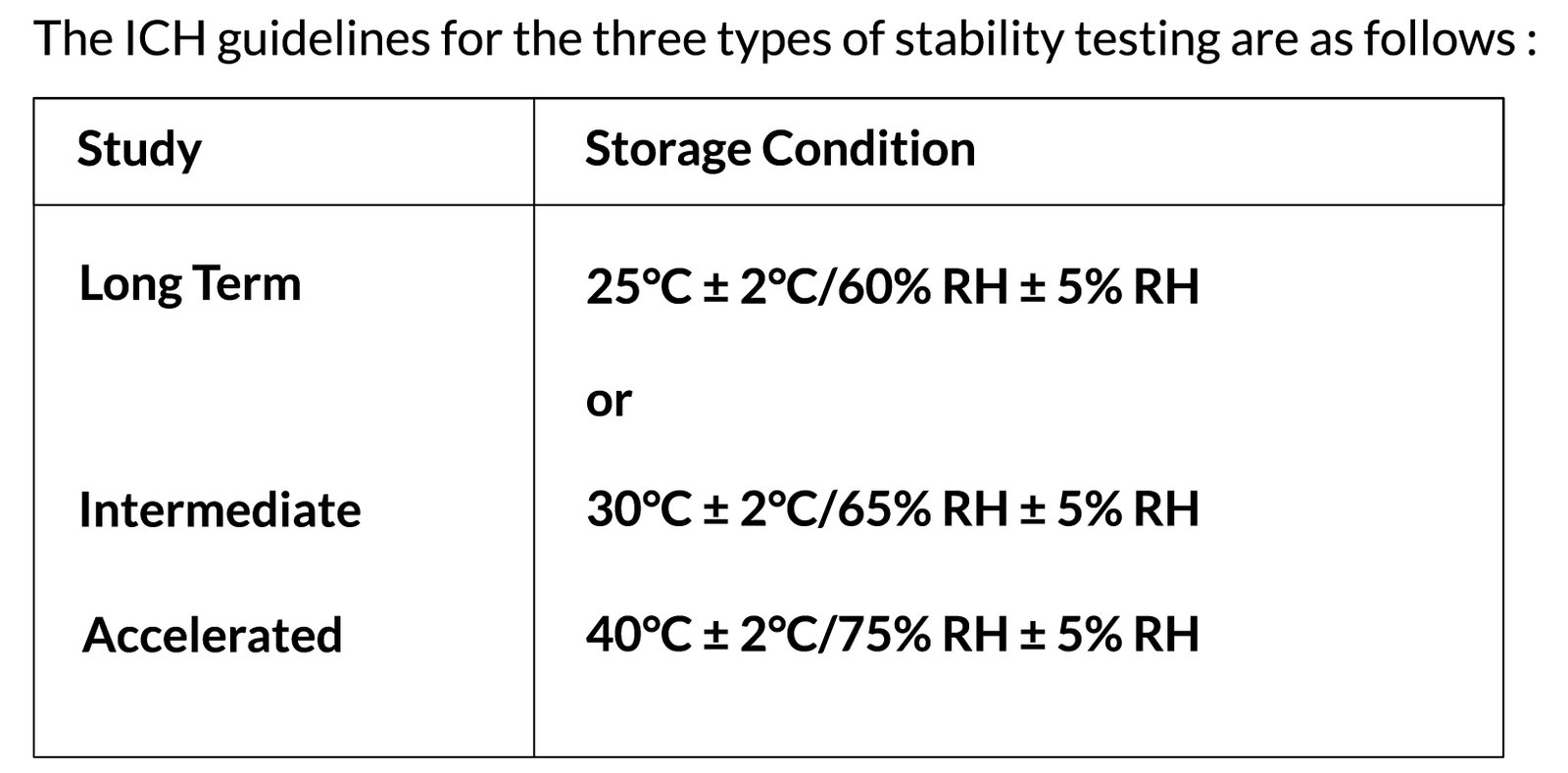
The ICH sets criteria for stability testing based on three different types of studies: long term stability studies (also referred to as real time), intermediate studies, and accelerated studies.

STABILITY CHAMBERS: ALLY ONE MODEL NO.: AO/SC-300
CAPACITY:3000L
To store the samples at the designated condition for the duration of the study, To store the samples at the designated condition for the duration of the study, temperature controlled humidity chambers are utilized to precisely control and maintain the desired set point. Failure to maintain the required condition for a stability study may mean a loss of several months or years of work, so it is of high importance to ensure that the equipment is built to exact specifications and will consistently hold the needed temperature and humidity levels.
We at Inducare Pharmaceuticals and Research foundation provides the infrastructure and facility for the stability testing of the Drug Substances and Drug Product. IPRF offers comprehensive stability studies according to ICH guidelines. We have installed dedicated stability chambers for each interval having 3000L capacity for the stability studies.
The stability chamber is a modular structure panel with internal stainless-steel body and external stainless-steel body insulated with 80 mm thick PUF insulation. The base of the chamber is provided with heavy-duty stainless-steel flooring to carry load of the equipment. The equipment maintains the temperature and humidity as per the set point in the HMI within the specified limits.
The temperature and humidity are controlled by PLC, controlling sensor and DTC through PID auto tuning. For continuous monitoring purpose, monitoring sensors are installed. Based on printing the same data is stored in PLC which transfers to the PC via Ethernet for data management.
